Industrialization advancement led to an increasing burden on the environment by releasing large quantities of hazardous wastes [1], [2]. Industrial effluents are mainly composed by environmental relevance metals (ERM) among others metalloids and organic contaminants that inflicted serious damage on ecosystems [3], [4]. Environmental relevance metals’ pollution is a serious concern due to the negative impact at even very small concentrations [5]. It is a direct consequence of processes as tannery [6], electroplating [7], dyeing or mining [8], and also of agriculture, sewage sludge [9] or waste treatment plants [10]. Certain industrial activities such as electroplating, mining, and metal-processing operations have been increasing in the last decades according to the improvement needs of specific characteristics in metallic products, such as oxidation resistance, conductivity, hardness, brightness, thermal and mechanical resistance among others. As consequence of these operations, high volumes of effluents containing ERM are generated which, if not being properly treated, could cause an irreversible harm to the environment. Recent studies have established that the long-term use of untreated wastewater from industrial sources can adversely affect water quality [11], making it unsuitable for human consumption [12], [13]. Being non-biodegradable [14], ERM tend to persist in the environment, leading to bioaccumulation in tissues and biomagnification along with the trophic levels [15]. Various reports have demonstrated that industrial wastewater contains metals beyond permissible limits [12]. In Buenos Aires - Argentina, the competent entity Autoridad de Cuenca Matanza Riachuelo (ACUMAR) stablished the discharge limit for copper of ≤ 2.0 mg/L in liquid effluents [16].
Copper (Cu) is an essential micronutrient for plants and other organisms because its ability to cycle between Cu(II) and Cu(I) [5]. These species are essential for biological functions serving as a cofactor for enzymes that are involved in processes such as photosynthesis and respiration. However, at high levels of copper this property makes it potentially toxic because the transitions between Cu(II) and Cu(I) can result in the generation of reactive oxygen species (ROS) such as superoxide and hydroxyl radicals, often causing oxidative stress which in turn may convey to membrane disintegration, lipid peroxidation and DNA damage [17]. Also, excessive Cu(II) intake leads to severe mucosal irritation and corrosion, stomach upset or ulcer, wide spread capillary, hepatic and renal damages (for example Wilson’s disease leading to brain damage), central nervous system irritation followed by depression, gastrointestinal irritation and possible necrotic changes in the liver and kidney [18], [19]. Besides, there are reported studies of copper toxicity in microorganisms [20].
Since there is a growing awareness of the persistence in nature, the ability to be incorporated and the ERM harmful effects on biodiversity, then a special interest to develop innovative technologies to remediate polluted environments is increasing. Several conventional techniques have been applied to remove ERM from wastewater [9]. Most of them are chemical and physical methods such as adsorption with activated carbon, oxidation, ion exchange, membrane filtration and chemical precipitation; but these practices can be expensive, energy costly, and need time and constant maintenance [17]. Besides, these methods have been associated to a secondary pollution by a toxic sludge formation difficult of being disposed of [21]. Furthermore, most of these techniques are more expensive and/or ineffective at very low ERM concentrations, specifically below 100 mg/L. Consequently, specific treatments are required to decrease metal concentration in this kind of wastes to comply with current regulations and to prevent environmental disturbances. In addition, some treatments could promote metal recovery for their recycling which represents an economically profitable process [15].
In the past two decades, there have been recent advances in biological remediation techniques. Microorganisms found in ERM polluted sites play a crucial role in converting toxic metals into non or less toxic forms [22]. Since microorganisms have developed survival strategies in those habitats, their different detoxifying mechanisms such as bioaccumulation, biotransformation, biomineralization and biosorption can be applied either ex situ or in situ to design economical bioremediation processes [23]. When effluents contain metal concentrations less than 100 mg/L, the use of biological methods is preferred [24] because their effectiveness and low cost [25], [26]. The ideal outcomes of biological treatments are that the polluted sites can be restored to their original conditions without any other harmful effect on the environment [27]. These are innovative technologies available for metal removal in wastewaters and their effectively restore polluted environments in an eco-friendly approach with low cost compared to conventional techniques.
This work was developed considering the environmental problematic previously mentioned and the need to find a sustainable alternative way to eliminate copper from effluents. The aim of this work was to study the interaction of free (BL) and immobilised on inert matrices (BI) cells of Pseudomonas veronii 2E with Cu(II), and to design effluent-biotreatment systems through sorption/desorption cycles, both in batch and continuous reactors. Thus, this work was carried out in two separated steps. First, the best conditions in which the free biomass can adsorb as much metal as possible in batch reactors were studied. Second, the established conditions to eliminate Cu(II) from a model effluent by the immobilised biomass in continuous reactors were applied. Metal desorption was also studied in each step to evaluate potential copper recovery for recycling.
Figure 1 represents the methodology that was carried out in this work as a flowchart.
Flowchart of the applied methodology
The most relevant assays carried out in this work using free biomass are detailed below.
30 mL of early stationary phase cultures (Absorbance at 600 nm, A600nm=1.1–1.8) performed in M9 broth (per litre: potassium phosphate dibasic (K2HPO4) 6 g; potassium phosphate monobasic (KH2PO4) 3 g; ammonium chloride (NH4Cl) 1 g; sodium chloride (NaCl) 0.5 g; 2% yeast extract 2.50 mL; 1 M calcium chloride (CaCl2) 1 mL; 1 M magnesium sulphate (MgSO4) 2.2 mL) supplemented with 2% glycerol or in PYG broth (per litre: peptone 5 g; yeast extract 2.5 g and glucose 1 g) were centrifuged (3000 g, 20 min). Pelleted cells were washed and resuspended in an adequate volume of ultrapure water (18 MΩcm, Millipore). The resulting suspension contained living biomass at a concentration of approximately 3 g dry weight/L.
A general biosorption assay was designed for testing metal retention mediated by bacteria under non-growth conditions. For that purpose, 5 ml cellular suspension was exposed to Cu(II) concentrations in the range from 0 to 2 mM, 0.1 mL 1 M 2-[N-morpholino]ethansulfonic acid buffer (MES, pH= 5.5) and ultrapure water (18 MΩcm, Millipore) to a final volume of 10 mL and incubated at 32 ºC and 120 rpm during 24 h. Suspensions were centrifuged (4942 g, 20 min) to obtain cell-free supernatants. Cu(II) equilibrium concentrations were measured in each supernatant after incubation and compared to cell–free control biosorption mixture.
Bacterial cells were harvested from cultures carried out in two different media (M9 and PYG) and exposed to 0.5 and 1 mM Cu(II) at different temperatures (25 y 32 ºC) in experimental conditions previously established (pH 5.5; 32 ºC and 120 rpm) to explore metal biosorption kinetics. Samples were collected at different times and centrifuged (6300 g, 15 min) to quantify remaining Cu(II).
The methodology used for the choice of an adequate matrix, the study of the biomass-immobilisation and its interaction with copper are detailed below.
The matrices that were chosen for cell immobilisation were pumice (PP), loofa sponge (EV) and diatomite (PS), according to parameters such as a good adhesion, easy operation/regeneration, high porosity including also chemical, biological, mechanical and thermal stability. Each support was processed as follows: cut, washed, dried, weighed and sterilized (autoclaving at 1 atm, 121 ºC for 20 min) [28].
The immobilisation was carried out in a batch reactor. For this purpose, 90 mL of M9 medium supplemented with 2% glycerol and the matrix were placed in a 500 mL glass flask. Then, 10 mL of a bacterial culture at early stationary growth phase was inoculated. The system was incubated at 32 ºC with slow agitation in an orbital shaker for 16 days, renewing the medium every 2-3 days under aseptic conditions.
To study metal sorption in a batch reactor the BI was exposed to a model effluent containing 1mM Cu(II), with a final volume of 50 mL (not including the volume occupied by BI), at pH 5.5 adjusted with 0.5 ml 1 M MES buffer. The reactor was incubated at 32 ºC with slow agitation and samples were taken at different times during 5 h and a last one at 24 h. The Cu(II) concentration was measured in each supernatant. The colonization of P. veronii 2E on each matrix was assessed by scanning electron microscopy (SEM).
To explore metal sorption in a continuous reactor, BI was placed in a column to which the model effluent was added with the same Cu(II) concentration as in the batch reactor described above. An upward flow was input using a pump (Watson Marlow 505s) located in the base of the reactor, and the treated effluent was collected by another pump in the upper part of the reactor. Both pumps worked with 12 mL/h flow rate. The effluent inside the reactor was continuously homogenized by a magnetic stirrer, meanwhile the temperature was kept constant at 32 ºC. The final effluent volume was 50 mL with the BI included. Samples were taken at different times during 5 h and a last one at 24 h. Two models of continuous reactors were built, one operated with recirculation (Rrc) and the other with 3 continuous reactors connected in series (Rs). Both systems were treated with 200 mL of the same effluent under identical conditions already mentioned.
For metal desorption, hydrochloric acid (HCl) was chosen as desorbent based on reported bibliography [29]. It was tested between 0.075 to 1.5 M HCl in the smallest possible volume in which Cu(II) recovery was maximal.
Once Cu(II) sorption by BL was finished, the batch reactor content (Vf = 10 mL) was centrifuged at 6300 g during 20 min to separate bacteria from the model effluent. Then, cells were resuspended in 2.5 mL of 0.075, 1.0 and 1.5 M HCl, and incubated for 30 min at 32 °C and 110 rpm. This procedure was repeated two more times, resulting in three total desorption cycles. In addition, Cu(II) desorption cycles by BL were repeated with other volume conditions (1, 2 and 2.5 mL) of 0.075 M HCl. The amount of Cu(II) in the supernatants was measured and compared to biomass-free control.
Cu(II) desorption by BI was carried out in both batch and continuous reactors. Once the metal sorption was concluded, the reactors were emptied, 17 mL of 0.075 M HCl were added and then incubated at 32 ºC with slow agitation for 30 min, repeating this procedure twice. Then, Cu(II) in the supernatants was quantified.
As the work volume with the model effluent in the Rrc and Rs was 200 mL, the desorption was performed in 80 mL of 0.075 M HCl as a final volume.
Cu(II) concentration in the supernatants was spectrophotometrically measured using the Bicinchoninic acid method [30].
The most relevant results of this research after following the already mentioned methodology are shown below.
Considering the chemical structure of bacterial envelopes and biofilm matrix exopolymers, carboxylate, hydroxyl, thiol, phosphate, amine and sulphate groups are exposed to cellular microenvironments. These functional groups are able to interact with metal ions for example. Their binding capacity allows bacterial surfaces to be considered as a conjunction of ligands, which can decrease the amount of bioavailable metal - Cu(II) in this case- and consequently reduce the toxic effects on cells. Being functional groups, their complexing capacity depends on the pH of the environment, so it is essential to keep its value under control [18]. This is the basis of the mechanism that contributes to biosorption, a term that refers to a process of metal uptake by biological matrices. Both the pH regulation and the units for the expression of concentration are essential to a correct interpretation of the results obtained [31].
The Langmuir model assumes that adsorption process takes place in a monolayer, that all adsorption sites are identical and that no changes in adsorption free energy are observed. According to this, when adsorption follows the Langmuir model, the number of occupied sites, q, is given by eq. (1):
(1)
(2)
Langmuir's linearized model is shown in eq. (2), where qmax is related to the total number of adsorption sites, Ceq is Cu(II) final equilibrium concentration in supernatants and Kd is the equilibrium constant for the dissociation of the surface complex. The number of occupied sites, q, can be calculated as indicated by eq. (3):
(3)
where Ci is the initial Cu(II) concentration, V is the total volume of the biosorption mixture assayed and m is the total biosorbent mass as dry weight.
The Freundlich isotherm is shown in eq. (4) and the linearized model in eq. (5):
(4)
(5)
where q is mmol Cu(II)/g dry weight biomass, Ceq is Cu(II) concentration measured after equilibration, Kf is related to the adsorption capacity, and n is an indicator of the adsorption intensity. In this empirical model, all sites on the surface are not considered equal. Adsorption becomes progressively more difficult as more and more adsorbate accumulates. It is assumed that once the surface is covered, additional adsorbed species can still be accommodated, leaving no possible prediction of maximal monolayer adsorption. In other words, the Freundlich model does not consider the existence of a monolayer and a maximal adsorption capacity as the Langmuir model does [32].
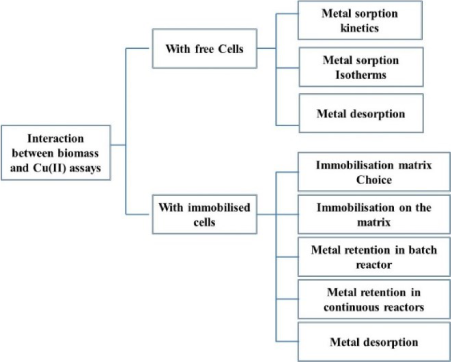
The metal sorption isotherms by the BL, under the working conditions mentioned in methods, are shown in Figure 2a, Figure 2b, Figure 2c. It can be observed in Figure 2a that in a range of 0 to 2.00 mM Cu(II), the sorption was between 40.0% - 79.2%, and 20.0% - 54.8% by cells harvested in M9 and PYG medium respectively. A maximum sorption capacity (qmax) of 0.523 and 0.134 (mmol/g biomass) by BL harvested from M9 and PYG medium was observed respectively.
In Figure 2b, Figure 2c is shown the linearization according to Langmuir and Freundlich models respectively. On the one hand it can be seen from the Figure 2b, R2 values are 0.9868 and 0.9368 for Langmuir model by cells taken from M9 and PYG respectively. On the other hand, R2 values for Freundlich Model 0.7741 and 0.7582 are shown by cells taken from M9 and PYG respectively.
Cu(II) biosorption isotherms in a range from 0 to 2.00 mM, in a batch reactor, for cells harvested from M9 medium in red and PYG medium in green
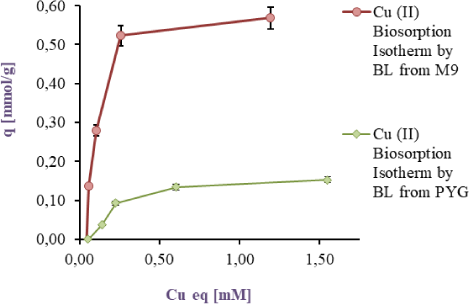
The results of the biosorption isotherms linearization of Cu(II) according to Langmuir model, in a range from 0 to 2.00 mM, in a batch reactor, are showed for cells harvested in M9 medium in red and for PYG medium in green
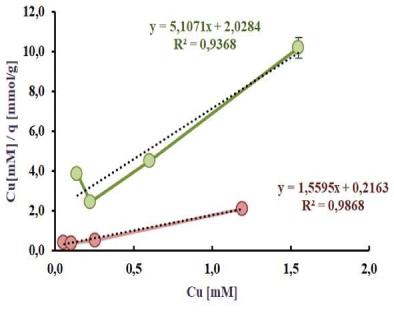
The results of the biosorption isotherms linearization of Cu(II) according to Freundlich model, in a range from 0 to 2.00 mM, in a batch reactor, are showed for cells harvested in M9 medium in red and for PYG medium in green
As can be seen from the Figure 2b and Figure 2c, Langmuir model best fitted to the Cu(II) sorption. In addition, the Langmuir constant Kd was 0.139 and 0.397 mM, while the qmax was 0.641 and 0.196 mmol/g respectively in each culture condition mentioned above.
This indicates that bacteria harvested from M9 medium behaved as better adsorbents than those taken from PYG medium. The differences observed in the Cu(II) sorption isotherms for BL may be related to the Pseudomonas veronii 2E ability to produce exopolymers, whose composition depended on the components of culture media besides the physical factors [33].
To be considered a good sorbent, a system usually is associated to low Kd and high qmax values. The parameters according to Langmuir model (Kd, qmax) were determined by the linear regression method and were compared to other reports as summarized in Table 1. Kd was similar to those obtained with the same microorganism with Cd(II) [34] and Aspergillus niger with Cu(II) [35], while higher values were obtained with P. veronii 2E with Zn(II) [32], Bacillus subtilis[19] and Pseudomonas putida with Cu(II) [36].
Comparison of reported Langmuir parameters describing biomass sorption-capacities
References |
Kd |
Qmax |
|||||
|---|---|---|---|---|---|---|---|
Metal |
Microorganism |
mg/L |
mM |
mg/g |
mmol/g |
R2 |
|
Cu(II) |
P. veronii 2E |
8.833 |
0.139 |
40.729 |
0.641 |
0.987 |
|
[34] |
Zn(II) |
P. veronii 2E |
3.007 |
0.046 |
12.945 |
0.198 |
0.853 |
[32] |
Cd(II) |
P. veronii 2E |
11.353 |
0.101 |
26.641 |
0.237 |
0.907 |
[35] |
Cu(II) |
A. niger |
8.19 |
0.13 |
17.60 |
0.28 |
0.998 |
[19] |
Cu(II) |
B. subtilis |
0.010 |
0.0002 |
100.70 |
1.58 |
0.994 |
[36] |
Cu(II) |
P. putida |
0.039 |
0.0006 |
0.858 |
0.014 |
0.995 |
Interestingly, the qmax observed was one of the highest values obtained, indicating that Pseudomonas veronii 2E could be a suitable sorbent for Cu(II).
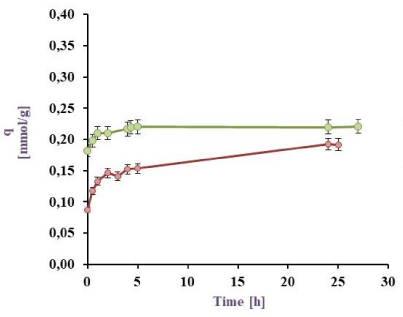
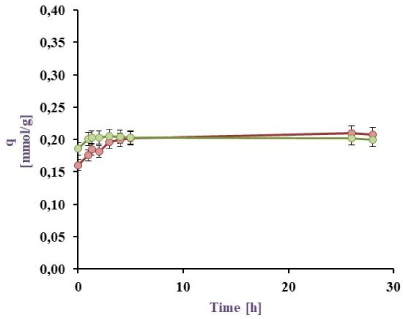
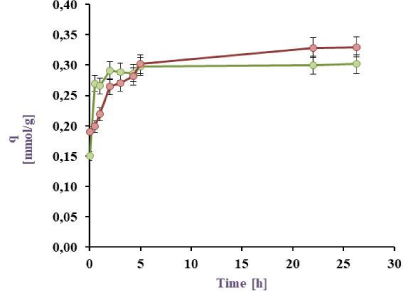
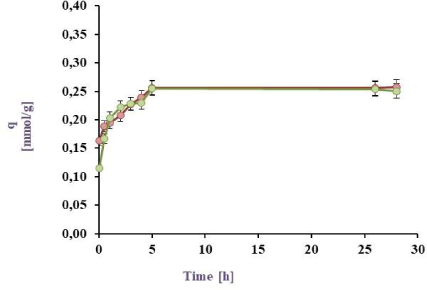
The results for the Cu(II) sorption kinetics with cell biomass (BL) obtained under different culture conditions are shown in Figure 3a, Figure 3b, Figure 3c, and Figure 3d. It can be observed that for the 0.5 mM Cu(II) model effluent the maximal sorption by the BL harvested from M9 was 88.6 (25 ºC) and 86.3 (32 °C) meanwhile from PYG 89.5% (25 ºC) and 88.9% (32 °C). Besides for Cu(II) 1 mM model effluent, maximal values were 79.7 (25 ºC) and 86.9 (32 °C) from M9 meanwhile 77.7% (25 ºC) and 81.6% (32 °C) from PYG. These values are higher than those reported in previous studies with Pseudomonas veronii 2E sorption (82.0 and 76.8% for Zn(II) and Cd(II), respectively) [31], [32]. Cu(II) sorption by P. veronii 2E was even higher than the 60-70% observed at the same pH by Tsekova et al. [35].
The results for the 1 mM Cu(II) sorption using cells harvested from M9 medium at 32 °C were higher than at 25 °C (q= 0.33 mmol/g vs. 0.26 mmol/g). It was also observed that bacteria previously grown in M9 medium indicated metal saturation at higher concentrations (0.33 mmol Cu(II)/g dry weight cell) than those developed in PYG medium (0.30 mmol Cu(II)/g dry weight cell). Liu et al. [19] studied the copper sorption by Bacillus subtilis immobilised into chitosan beads, finding that copper sorption increased with temperature. In this work, the same trend was observed for 1 mM copper effluents: an increase of the temperature increased the copper sorption a 7.1% and a 3.9% by cells obtained from M9 and PYG medium respectively. Moreover, in effluents containing 0.5 mM Cu no appreciable differences in metal sorption were observed with a temperature increase. As consequence of these results, the conditions for the following experiments were established: 32 °C, cell biomass harvested from M9 medium and a 1 mM Cu(II) model effluent.
Cu(II) Biosorption in a batch reactor from a 0.5 mM model effluent: BL harvested from PYG medium (in green) and M9 medium (in red) at 32 ºC
Cu(II) Biosorption in a batch reactor from a 0.5 mM model effluent: BL harvested from PYG medium (in green) and M9 medium (in red) at 25 ºC
Cu(II) Biosorption in a batch reactor from a 1 mM model effluent: BL harvested from PYG medium (in green) and M9 medium (in red) at 32 ºC
Cu(II) Biosorption in a batch reactor from a 1 mM model effluent: BL harvested from PYG medium (in green) and M9 medium (in red) at 25 ºC
The most relevant results obtained for Cu(II) sorption assays under the optimal working conditions already mentioned in batch and continuous reactors are summarized below.
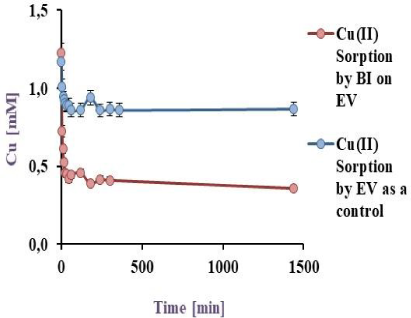
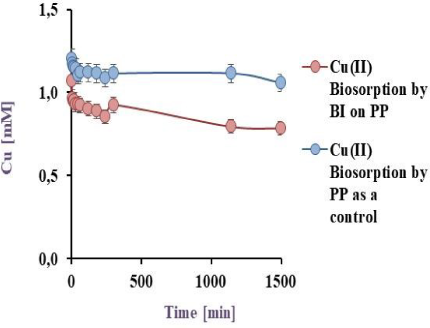
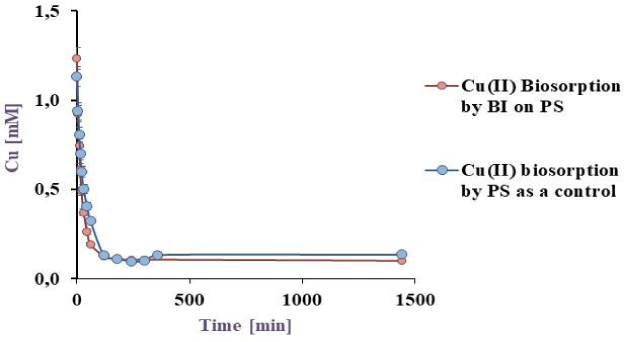
50 mL of a model effluent containing Cu(II) was exposed to BI on EV, PP and PS, the pH was adjusted to 5.5, at 32 °C for 24 h in a batch reactor, the results are shown in the Figure 4a, Figure 4b and Figure 4c.
Cu(II) biosorption by BI on EV at 32 °C under agitation
Cu(II) biosorption by BI on PP at 32 °C under agitation
Cu(II) biosorption by BI on PS at 32 °C under agitation
As can be seen in Figure 4a and Figure 4b, the sorption obtained by BI and the matrix (71.4 and 33.9%) was higher than the cell free matrix (27.4 and 11.4%) for EV and PP respectively, evidencing the bacterial influence on copper-sorption. Similar results were obtained by Verma et al. [18] who studied copper sorption by Penicillium citrinum immobilised on alginate. In this study, the sorption value obtained was 76.2% but using a six times lower metal concentration.
Figure 4c revealed a metal sorption by BI-matrix and the biomass-free matrix of 92.1 and 81.9%, respectively. Although the highest sorption obtained, PS were totally disintegrated at the end of the experiment remaining as a dust in suspension, which makes it unsuitable for the biotreatment. In addition, in all cases sorption occurred surprisingly fast with practical significance for a bioremediation process.
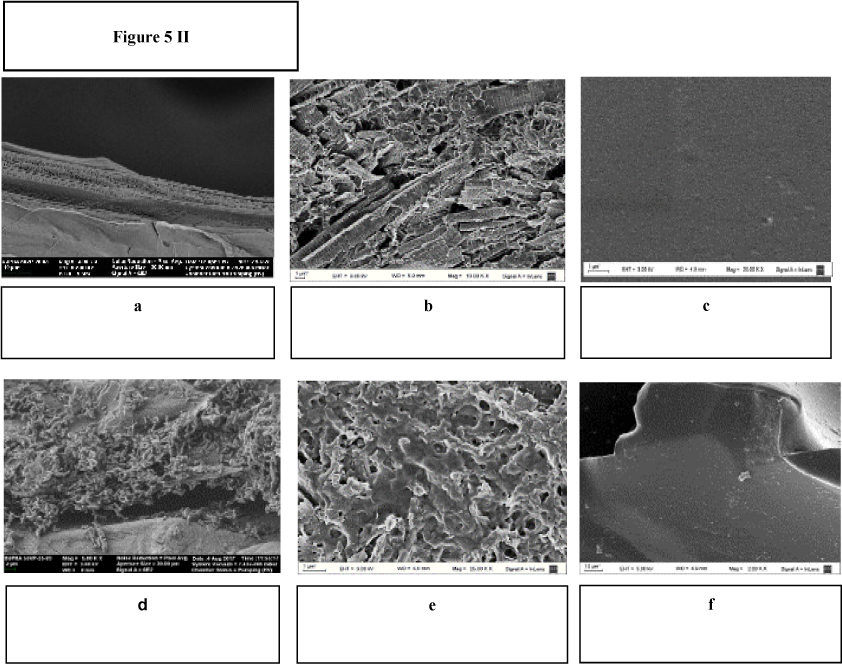
Bacterial development on the matrix surfaces was specifically studied for a more complete understanding of the biosorption differences found. Figure 5Ia, Figure 5Ib, Figure 5Ic, Figure 5Id, Figure 5Ie and Figure 5If show the macroscopic changes of the surfaces before and after the bacterial immobilisation.
Macroscopic changes of the matrix: a) EV, b) PS and c) PP before bacterial immobilisation, d) EV, e) PS and f) PP with the biomass immobilised
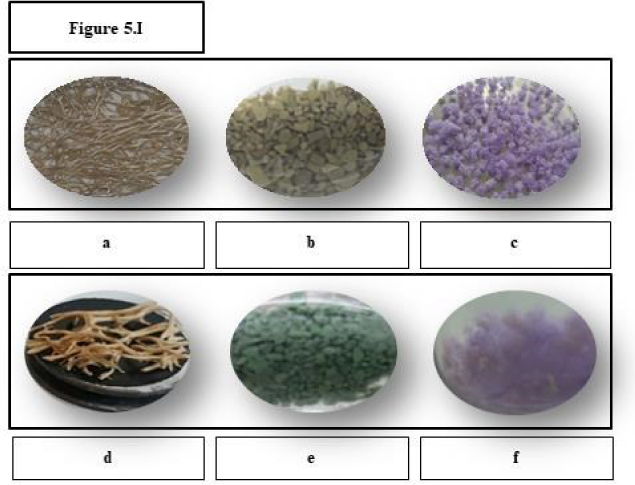
Figure 5II shows the images of bacterial colonization on the different matrices captured by scanning electron microscopy (SEM). Figure 5IIa, Figure 5IIb and Figure 5IIc correspond to the cell-free materials and Figure 5IId, Figure 5IIe and Figure 5IIf show the bacterial development on EV, PS and PP respectively. The SEM microphotographs confirmed that bacteria could develop a widely spread biofilm on the loofa sponge rather than the other surfaces. These image analyses explained the great differences found in the biosorption results. In pumice, the bacteria seemed to be able to adhere only to the surface folds, explaining the lower biosorption yields. Finally, as diatomites presented more porosity, the bacterial growth appeared as a thin layer on the surface. The sorption of this last system came mostly from the matrix rather than from biomass itself. In addition, the diatomite particles turned to a fine dust in suspension during the experiments, making this method useless for the established purposes. Therefore, Cu(II) biosorption assays were continued only with BI on loofa sponge.
Effectively, EV was the most adequate matrix to immobilise P. veronii 2E, since bacteria could colonize the entire matrix surface with a high retention (71.4%). In addition, these sorption values are higher than obtained by other authors as Tsekova et al. (34.0%) [35].
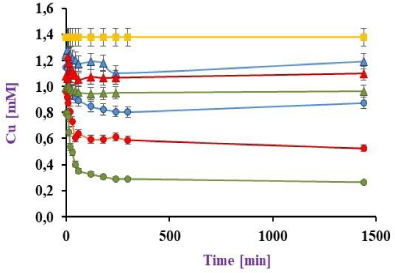
A continuous reactor with recirculation (Rrc) was constructed and, by other side, 3 series reactors were designed to continuously operate (Rs). Both were filled with P. veronii 2E immobilised on EV and exposed to 200 ml of a 1 mM Cu(II) model effluent under the conditions already mentioned. The results are shown in Figure 6a and Figure 6b, where for Rrc: 34.5 and 78.3 % of Cu(II) retention was observed by the BI on the EV, which 27.1 and 50.9 % corresponded only to the BI at 1 and 9 days respectively. Maximal copper sorption was initially achieved up to 5 h. For Rs, a 56.0 % of metal retention at 24 h was obtained, similar results than those reported by Liu et al. [19] with sorption values of 55.4 % but using 0.79 mM initial Cu(II).
BI analyses by SEM: a) EV, b) PS and c) PP without adhered biomass and d) EV, e) PS and f) PP with the biomass immobilised
Cu(II) biosorption by BI on EV, at 32 ºC in a final volume of 200 ml for 9 days, in a continuous bioreactor with recirculation. The metal concentration measured in the supernatant is observed in red by BI on the matrix and green by only the matrix as a control
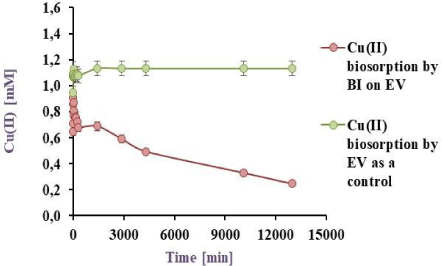
Cu(II) biosorption by BI on EV, at 32 ºC in a final volume of 200 ml for 9 days, in a continuous bioreactor with recirculation. The metal concentration measured in the supernatant is observed in red by BI on the matrix and green by only the matrix as a control
Although a higher sorption was obtained by BI in a Rs, this system contained more biomass than in Rrc. To evaluate which system was more efficient, a parameter q' was compared in both, Rrc and Rs, at 24 h. Thus, q' is the metal sorption normal-capacity of a system, and it is calculated according to equation 6:
(6)
where q is the number of occupied sites and nt is the initial amount of Cu(II) in mmol.
In this way, it is possible to compare Cu(II) sorption normal-capacity of each system regardless of the amount of biomass and metal that it works with. The q' results were 0.34 and 0.43 g-1 by Rs and Rrc respectively.
To a broad understanding about Rs performance, a mass balance was evaluated taken into account that the metal quantification was focused on soluble Cu(II). For this purpose, residual Cu(II) was measured at each reactor outlet, showing a total of 44.0% of the initial Cu(II) concentration. Also, at the same conditions, the BI in each reactor different sorption percentages were registered according to the effluent serial dilution. Integrating mass balance with q' values, it could be concluded that the recirculating reactor is more efficient than the 3 reactors connected in series one sequentially after the other. An interesting point to deepen in its analysis would be the determination of Cu(II) sorption mediated by biomass in the reactors connected in series, adding an effluent recirculation from 1 to 9 days and to compare with Rrc.
For desorption assays, the information based on the results obtained in previous works was compiled. As example, Akhtar et al. [29] studied different matrices to eliminate nickel from a model effluent, obtaining the highest metal recovery values using HCl as desorbent. Therefore, in this work HCl was tested at different acid concentrations as desorbent, combining the smallest possible work volume with the highest metal desorption.
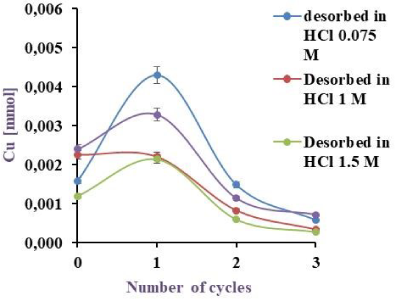
To evaluate the best desorption method, different HCl concentrations (0.075, 0.1, 1.0 and 1.5 M) were tested in a fixed work volume (2.5 ml), choosing the acid concentration at which the maximal Cu(II) recovery was obtained. The results are shown in Figure 7a for 3 cycles of half an hour each, monitoring in which instance the metal was completely desorbed. The results are shown in Figure 7b.
Cu(II) Desorption by BL with HCl 0.075, 0.1, 1.0 and 1.5 M
Cu(II) Desorption by BL in HCl 0.075 M
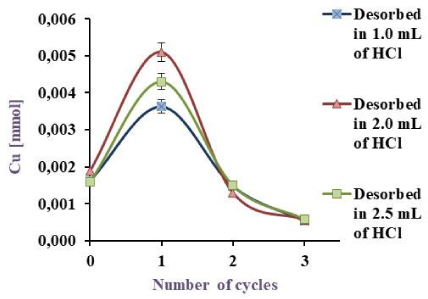
As can be extracted from Figure 7a, 20.7, 23.8, 36.0 and 42.7% desorbed in the first cycle and the total desorption was 29.2, 36.5, 56.4 and 62.8% for 1.5, 1.0, 0.1 and 0.075 mM HCl respectively. As shown in Figure 7b, metal desorption was 36.5, 53.0 and 47.7% in the first cycle with a total desorption of 56.8, 72.1 and 62.9% using 1, 2 and 2.5 ml of desorbent respectively.
For Cu(II) desorption assays, 50 ml of a model effluent was exposed to the BI on each matrix in a batch reactor during 24 h. Then, the reactor content was emptied, and 17 ml of HCl was added and metal concentration in supernatants was measured after 30 min.
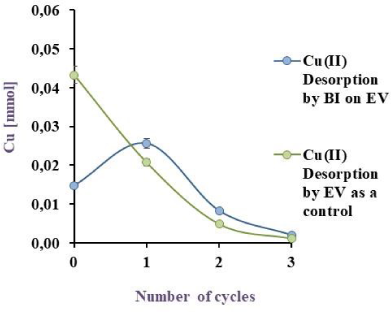
Cu(II) Biodesorption by BI on EV in blue and its corresponding control (EV) in green EV to 32 ºC, under agitation
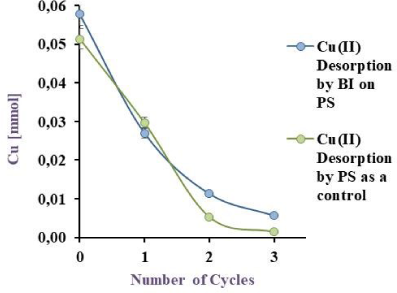
Cu(II) Biodesorption by BI on PS in blue and its corresponding control (PS) in green EV to 32 ºC, under agitation
Cu(II) Biodesorption by BI on PP in blue and its corresponding control (PP) in green EV to 32 ºC, under agitation
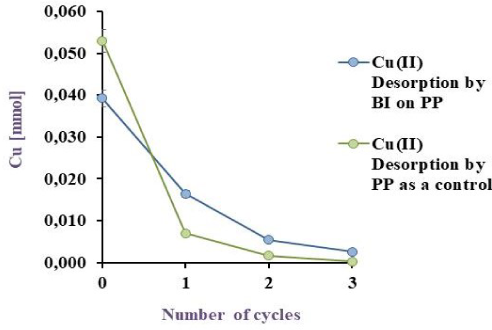
Figure 8a, Figure 8b and Figure 8c illustrate the obtained results, with a desorption of 76.6, 81.6 and 51.2% for BI on EV, PP and PS respectively. Akhtar et al. [29] obtained 98% of Ni(II) desorption (10 mg/L) by Chlorella sorokiniana immobilised on loofa sponge while results in this work with the same matrix showed a 76.6 % of Cu(II) desorption. Although the % of metal recovery in this study is lower than the reported by Akhtar et al. [29], the initial copper concentration was six times higher confirming that this desorption agent is suitable for this system. Unfortunately, in literature [36] metal desorption was carried out, but with insufficient data to compare to and to ensure the viability of the method developed in this work for Cu(II).
For the Rrc and Rs, as the volume of model effluent was 200 ml, desorption was performed in a final volume of 80 ml of 0.075 M desorbent, discriminating the biomass free matrix desorption. These results are shown in Figure 9 where it can be observed that at 30 min desorption was 80.9%, being almost total at 60 min (97.2%).
Cu(II) Biodesorption by BI on EV in blue and by only the matrix, in green, as a control in a batch with recirculation at 32 °C
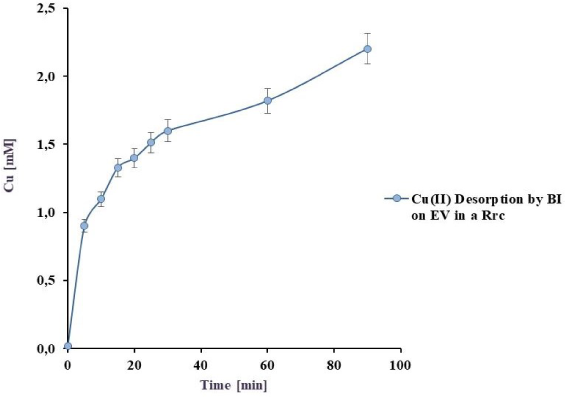
This desorption value is much higher than the obtained with BL (72,1%). For Rs, results were compiled in Table 2, reporting that the exclusive BI desorption on EV was 38.5% while a 43.3% was recovered from only the matrix.
Cu(II) sorption/desorption by BI on EV in Rs and its corresponding control at 32 °C
Adsorption |
Cu Initial [mmol] |
Cu adsorbed[mmol] |
Cu desorbed [mmol] |
Cu(II) percentage adsorbed |
Cu(II) percentagedesorbed |
|---|---|---|---|---|---|
by BI + EV |
0.276 |
0.193 |
0.074 |
69.9 |
38.5 |
by EV (control) |
0.267 |
0.104 |
0.045 |
38.9 |
47.3 |
Evidently, low desorption percentages in Rs could be associated to an insufficient contact time between the system and the desorbent. This conclusion is based on the results obtained in Rcr, where BI on EV was able to desorb almost the complete Cu(II) retained (97.2%).
Cu(II) removal studies by Pseudomonas veronii 2E in this work contributed with extremely valuable data since it was proved that the designed systems removed high amounts of metal from model effluents in a very short time, enabling to desorb almost the total metal retained. In effluents with 1 mM Cu(II) the convenient working temperature was 32°C. In these conditions, Pseudomonas veronii 2E recovered an 86.9% of the metal when it was cultured in M9 medium. The BL could desorb a 72.1% of the metal using HCl. By immobilising the biomass, a high metal sorption (71.4%) was still achieved, facilitating the separation of the biomass from the effluent, minimizing costs and opening the possibility to reuse the system. Two continuous reactors were built to improve the process design, and it was proved that a reactor with effluent recirculation was more efficient than three reactors connected in series. Even more, metal adsorption value was 78.3% in a reactor with recirculation, desorbing almost the total metal retained (97.2%) in one hour which is desired when exploring metal recycling.
The developed biotreatment represents the necessary first step that would drive to a viable and efficient process to both eliminate Cu(II) from effluents and recover the metal for recycling. In real effluents metal ligands frequently appear, so a complexing capacity is expected to decrease metal availability among other interferences. Therefore, a major complexity of real effluents should not be disregarded, leading to their analysis and characterization for further studies.
There are still limitations in the design such as the slower rates in metal-removal compared with physical and chemical methods. However, the advantage of being more economical should not be disregarded since there is no dependence on an active bacterial metabolism supported by an economic matrix. Furthermore, this process would not generate secondary effluents as physicochemical procedures do, becoming an environmentally friendly and thus a sustainable alternative to efficiently remove copper.
We would like to thank the National Scientific and Technical Research Council -Argentina and the National Agency for Scientific and Technological Promotion (PICT 2014- 0964) for financing this research and the National University of General Sarmiento, who besides funding this study, allowed us to use its facilities to carry it out.
P.B thanks I. Busnelli and L. Martinez of the Integral Centre of Electronic Microscopy (CIME- CCT- Tucumán) for the microscopy photos used in this work.
- ,
A new strategy for heavy metal polluted environments: A review of microbial biosorbents. ,Int J Environ Res Public Health. , Vol. 14 ,pp 94 , 2017, https://doi.org/doi:10.3390/ijerph14010094 - ,
Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. ,Int J Environ Res Public Health. , Vol. 14 ,pp 1504 , 2017, https://doi.org/doi:10.3390/ijerph14121504 - ,
Bead-immobilized Pseudomonas stutzeri Y2 prolongs functions to degrade s-triazine herbicides in industrial wastewater and maize fields. ,Sci Total Environ. , Vol. 731 ,pp 139183 , 2020, https://doi.org/doi:10.1016/j.scitotenv.2020.139183 - ,
Characterization of heavy metal toxicity in some plants and microorganisms—A preliminary approach for environmental bioremediation. ,N Biotechnol. , Vol. 56 ,pp 130-139 , 2020, https://doi.org/doi:10.1016/j.nbt.2020.01.003 - ,
Heavy Metal Toxicity and the Environment. ,Molecular, Clinical and Environmental Toxicicology Volume 3: Environmental Toxicology. , Vol. 101 , 2012, https://doi.org/doi:10.1007/978-3-7643-8340-4 - ,
Mecanismos moleculares de resistencia a metales pesados en las bacterias y sus aplicaciones en la biorremediación. ,Rev CENIC Ciencias Biológicas. , Vol. 41 (1),pp 67-78 , 2010 - ,
Bio-inspired materials for defluoridation of water: A review. ,Chemosphere. , Vol. 253 ,pp 126657 , 2020, https://doi.org/doi:10.1016/j.chemosphere.2020.126657 - ,
Bioremediation of toxic heavy metals (THMs) contaminated sites: concepts, applications and challenges. ,Environ Sci Pollut Res. , 2020, https://doi.org/doi:10.1007/s11356-020-08903-0 - ,
Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: Circular economy approach. ,Sci Total Environ. , Vol. 715 ,pp 136978 , 2020, https://doi.org/doi:10.1016/j.scitotenv.2020.136978 - ,
Bioremediation of cadmium polluted soil using a novel cadmium immobilizing plant growth promotion strain Bacillus sp. TZ5 loaded on biochar. ,J Hazard Mater. , Vol. 388 ,pp 122065 , 2020, https://doi.org/doi:10.1016/j.jhazmat.2020.122065 - ,
Characterization of Cd2+ biosorption by Pseudomonas sp. strain 375, a novel biosorbent isolated from soil polluted with heavy metals in Southern China. ,Chemosphere. , Vol. 240 ,pp 124893 , 2020, https://doi.org/doi:10.1016/j.chemosphere.2019.124893 - ,
Bioremediation options for heavy metal pollution. ,J Heal Pollut. , Vol. 9 (24), 2019, https://doi.org/doi:10.5696/2156-9614-9.24.191203 - ,
Bioremediation of heavy metals in food industry: Application of Saccharomyces cerevisiae. ,Electron J Biotechnol. , Vol. 37 ,pp 56-60 , 2019, https://doi.org/doi:10.1016/j.ejbt.2018.11.003 - ,
Ca-alginate as a support matrix for Pb(II) biosorption with immobilized biofilm associated extracellular polymeric substances of Pseudomonas aeruginosa N6P6. ,Chem Eng J. , Vol. 328 ,pp 556-566 , 2017, https://doi.org/doi:10.1016/j.cej.2017.07.102 - ,
Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. ,Bioresour Technol. , Vol. 303 ,pp 122886 , 2020, https://doi.org/doi:10.1016/j.biortech.2020.122886 - Anexo I. Tabla consolidada de límites admisibles de vertido de efluentes líquidos.Buenos Aires, Argentina, 2017, http://servicios.infoleg.gob.ar/infolegInternet/anexos/270000-274999/273042/res46.pdf, Published online.
- ,
Diatom mediated heavy metal remediation: A review. ,Bioresour Technol.123068 , Vol. 305 , 2020, https://doi.org/doi:10.1016/j.biortech.2020.123068 - ,
Biosorption of Cu (II) using free and immobilized biomass of Penicillium citrinum. ,Ecol Eng. , Vol. 61 ,pp 486-490 , 2013, https://doi.org/doi:10.1016/j.ecoleng.2013.10.008 - ,
Biosorption of copper(II) from aqueous solution by Bacillus subtilis cells immobilized into chitosan beads. ,Trans Nonferrous Met Soc China. , Vol. 23 ,pp 1804-1814 , 2013, https://doi.org/doi:10.1016/S1003-6326(13)62664-3 - ,
Heavy Metal Removal Investigation in Conventional Activated Sludge Systems. ,Civ Eng J. , Vol. 6 (3),pp 470-477 , 2020, https://doi.org/doi:10.28991/cej-2020-03091484 - ,
Emery, R.J.N. The Role of Phytohormones in Enhancing Metal Remediation Capacity of Algae. ,Bull Environ Contam Toxicol. , 2020, https://doi.org/doi:10.1007/s00128-020-02880-3 - ,
Bioremediation of heavy metals by microbial process. ,Environ Technol Innov. , Vol. 14 (100369), 2019, https://doi.org/doi:10.1016/j.eti.2019.100369 - ,
Potential use of algae for heavy metal bioremediation, a critical review. ,J Environ Manage. , Vol. 181 ,pp 817-831 , 2016, https://doi.org/doi:10.1016/j.jenvman.2016.06.059 - ,
Cadmium, zinc and copper biosorption mediated by Pseudomonas veronii 2E. ,Bioresour Technol. , Vol. 99 ,pp 5574-5581 , 2008, https://doi.org/doi:10.1016/j.biortech.2007.10.060 - ,
Comparative study on the biosorption of aluminum by free and immobilized cells of Bacillus safensis KTSMBNL 26 isolated from explosive contaminated soil. ,J Taiwan Inst Chem Eng. , Vol. 69 ,pp 61-67 , 2016, https://doi.org/doi:10.1016/j.jtice.2016.09.032 - ,
Bioremediation techniques–classification based on site of application: principles, advantages, limitations and prospects. ,World J Microbiol Biotechnol. , Vol. 32 ,pp 1-18 , 2016, https://doi.org/doi:10.1007/s11274-016-2137-x - ,
Enhancing cadmium bioremediation by a complex of water-hyacinth derived pellets immobilized with Chlorella sp. ,Bioresour Technol. , Vol. 257 ,pp 157-163 , 2018, https://doi.org/doi:10.1016/j.biortech.2018.02.060 - ,
Inmovilización microbiana: técnicas y usos en el tratamiento de residuos tóxicos. ,Sist Ambient. , Vol. 2 (1),pp 23-34 , 2008 - ,
Removal and recovery of nickel(II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana: Characterization studies. ,Journal of Hazardous Materials. , Vol. 108 ,pp 85-94 , 2004, https://doi.org/doi:10.1016/j.jhazmat.2004.01.002 - ,
A quantitative test for copper using bicinchoninic acid. ,Anaytical Biochemistry. , Vol. 226 ,pp 80-84 , 1995, https://doi.org/https://doi.org/10.1006/abio.1995.1194 - ,
Biosorption of Nickel (II) from Aqueous Solutions onto Pistachio Hull Waste as a Low-Cost Biosorbent. ,Civ Eng J. , Vol. 5 (2),pp 447 , 2019, https://doi.org/doi:10.28991/cej-2019-03091259 - ,
Zinc Biosorption by Microbial species for biotreatment processes, in Handbook of Metal-Microbe Interactions and Bioremediation ,pp 657-672 , 2016, https://doi.org/https://doi.org/10.1201/9781315153353-46 - ,
Chemical characterization of Pseudomonas veronii 2E soluble exopolymer as Cd(II) ligand for the biotreatment of electroplating wastes. ,Int Biodeterior Biodegrad. , Vol. 119 ,pp 605-613 , 2017, https://doi.org/doi:10.1016/j.ibiod.2016.10.013 - ,
Global journal of environmental science and technology Pseudomonas veronii 2E surface interactions with Zn(II) and Cd(II). ,Glob J Environ Sci Technol. , Vol. 1 (3), 2011 - ,
Biosorption of copper(II) and cadmium(II) from aqueous solutions by free and immobilized biomass of Aspergillus niger. ,Bioresour Technol. , Vol. 101 ,pp 1727-1731 , 2010, https://doi.org/doi:10.1016/j.biortech.2009.10.012 - ,
Biosorption of copper(II) from aqueous solutions using volcanic rock matrix-immobilized Pseudomonas putida cells with surface-displayed cyanobacterial metallothioneins. ,Chem Eng J. , Vol. 204-205 ,pp 264-271 , 2012, https://doi.org/doi:10.1016/j.cej.2012.05.029




